What is zinc carbonate?
Zinc Carbonate is a colourless or white solid that is found in nature forming the Smithsonite mineral, in which it can be alone or with other elements such as cobalt or copper, which give it violet or green colour respectively. ZnCO3 is almost insoluble in water, but it dissolves easily in dilute acids since the carbonate ion in an acidic medium forms carbonic acid (H2CO3), which is then converted into CO2 gas and water. It is used as an antiseptic in animal wounds and is sometimes supplied in the diet to avoid diseases caused by zinc deficiency.

What is zinc carbonate used for?
Zinc Carbonate has a lot of applications. The main ones are listed below:
In Veterinary Applications
It serves as an astringent, antiseptic, and topical wound protector in animals.
It also helps prevent diseases caused by zinc deficiency, which is why it is used as a supplement in the diet of some animals, provided that the amounts administered are within the standards established by health organizations.
Zinc carbonate is sometimes administered as a micronutrient to prevent disease in animals.
In Medical Treatments
This compound allows for obtaining some pharmaceutical products. It is applied to inflamed skin in the form of a powder or lotion.

As a Flame Retardant
It is used as a fireproof filler for rubbers and plastics that are exposed to high temperatures.
It protects textile fibres from fire. In the case of cotton textiles, it is applied to the fabric along with some alkali. This directly attacks the primary hydroxyl groups (–CH2OH) of the cellulose and converts them into sodium cellulose (–CH2ONa).
The breakdown of cellulose bonds by the alkali favours a greater penetrability of the chains of the compact cellulosic structure, which is why more ZnCO3 manages to enter the amorphous zone of this, and its dispersion is facilitated.
Some cotton fabrics may contain ZnCO3 in their fibres to make them fire-resistant. As a result, the amount of flammable gas that could be produced by fire is reduced.

To Separate Dangerous Minerals from Arsenic
Methods of separating arsenic minerals from sulfide rocks (such as galena, chalcopyrite, and pyrite) have been tested using ZnCO3. The mineral-rich in arsenic must be separated from the others because this element is a very toxic and poisonous pollutant for living beings.
To achieve this, the mixture of the ground rocks is treated with a solution of zinc sulfate and sodium carbonate at a pH of 7.5-9.0 and a xanthate compound.
The effectiveness of the formula is attributed to the formation of small ZnCO3 particles on the surface of the arsenopyrite, making it hydrophilic (related to water), so it cannot adhere to air bubbles and cannot float, precipitating and separating of the other minerals.
In Dental Treatments
Certain toothpaste based on zinc carbonate and hydroxyapatite nanocrystals are applied regularly to the denture. It reduces hypersensitivity more effectively than those based on fluorine.
ZnCO3 and hydroxyapatite nanocrystals have a size, shape, chemical composition and crystallinity similar to that of dentin so that the dentinal tubules can be closed with the application of these materials.
ZnCO3 -hydroxyapatite nanoparticles have been successfully tested to decrease sensitivity in whitened teeth. This type of toothpaste is useful after teeth whitening processes.
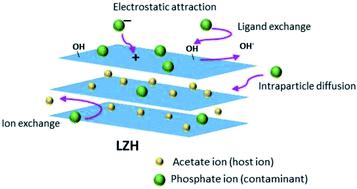
In The Recovery of Zinc from Residual Effluents
Synthetic waters rich in zinc ions discarded by electroplating processes can be treated by fluidized bed technology using sodium carbonate to precipitate ZnCO3.
By precipitating the Zn2+ in the form of the carbonate, its concentration decreases, the solid obtained is filtered, and the waters can be disposed of safely. The precipitated ZnCO3is of high purity.
Is zinc carbonate good for skin?
Zinc oxide and zinc carbonate, which are highly insoluble, are applied liberally to inflamed skin in the form of powder, calamine lotion, and the like. Zinc chloride, sulfate, and acetate have been used for their antiseptic, astringent, or caustic properties. Their use is limited by dificulty of local control, not by systemic effects. Zinc sulfate has been used as an emetic at an oral dose of 1000 or 2000 mg in a glass
of water . This dose intentionally produces a concentration in the water well above the threshold that may lead to vomiting if taken on an empty stomach, as sometimes happens accidentally when fruit juice or other acid drinks are stored in galvanized vessels.
What is production method of zinc carbonate?
The production method of zinc carbonate is mainly the compound decomposition method, which soon contains zinc or zinc oxide raw materials and sulfuric acid action, the crude zinc sulfate solution, by potassium permanganate oxidation, remove iron, manganese and other impurities, and then add zinc powder, after stirring can remove nickel, copper, cadmium and other impurities. It was then reoxidized with potassium permanganate to remove small amounts of manganese and iron. The refined zinc sulfate solution was combined with soda alkali to form alkaline zinc carbonate, and the reaction temperature was controlled by 46℃, pH6.8, and free alkali by 0.4% to 0.5%. The slurry material obtained by redecomposition, the filter cake is dry at 100℃, to the moisture content is below 2.5%, and then after fine grinding and screening, make alkali zinc carbonate finished products.
Is zinc carbonate A acid or base?
Zinc carbonate is a salt, not an acid or an alkali.
How to buy zinc carbonate?
At Qi Di Chem, we sell Zinc carbonate. The price of Zinc carbonate usually varies depending on the price of the raw material. For the latest price, please contact us and we will provide a quotation suitable for your country/region. We export worldwide, so you can contact us at email address mandy@czqidi.com for a free quote, or send a message on WhatsApp for a quick response +86-186-5121-5887.
If want to know more information,click QiDi Chem to find more details.
English
العربية
Français
Русский
Español
Português
Deutsch
italiano
日本語
한국어
Nederlands
Tiếng Việt
ไทย
Polski
Türkçe
አማርኛ
ភាសាខ្មែរ
Bahasa Melayu
ဗမာစာ
Filipino
Bahasa Indonesia
magyar
Română
Čeština
қазақ
हिन्दी
فارسی
Kiswahili
Slovenčina
Slovenščina
Norsk
Svenska
українська
Ελληνικά
Suomi
עברית
Latine
Dansk
اردو
বাংলা
Hrvatski
Afrikaans
Eesti keel
සිංහල
latviešu
Български
Hausa
íslenska
Kurdî
Lietuvių
isiZulu














