INTRODUCE:
Ammonium persulfateis an inorganic compound widely use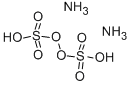 d in chemical experiments and ind
d in chemical experiments and ind
ustry. Its mole=cular formula is (NH4) 2S2O8, which is a white crystalline solid with strong oxidation properties. This compound is mainly prepared by reacting sodium persulfate with ammonia, and the reaction products are crystallized or purified under low temperature conditions.
INDUSTRIAL APPLICATIONS:
1. Polymerization reaction
Ammonium persulfate is often used as an initiator in polymerization reactions, promoting monomer polymerization and forming high molecular weight polymers. The triggering effect of ammonium persulfate is mainly achieved through its strong oxidizing properties. The following is a general mechanism of ammonium persulfate in polymerization reactions:
Selection of initiator: The selection of ammonium persulfate as the initiator is usually based on its high oxidation ability. It can release reactive oxygen species and participate in the initiation and expansion process of polymer chains.
Initiation process: Polymerization reactions typically include the initiation stage, expansion stage, and termination stage. In the initial stage, ammonium persulfate decomposes to produce active free radicals, which react with monomer molecules to form primary free radicals.
Polymerization process: Primary free radicals react with other monomer molecules to form longer polymer chains. This process continues during the expansion phase of the aggregation chain, resulting in the formation of larger aggregates.
Termination stage: The polymerization reaction will terminate at a certain moment, which can be achieved through different methods, such as the combination of two free radicals, depletion of reactants, etc. Termination of the reaction leads to the cessation of growth of the polymer chain.
Influencing factors: The efficiency of polymerization reactions and the properties of products are influenced by various factors, including the concentration of ammonium persulfate, reaction temperature, reaction time, and other conditions of the reaction system. The regulation of these factors can control the rate of polymerization reaction and the characteristics of the products.
2. Textile industry
Ammonium persulfate has important applications in the textile industry, mainly reflected in bleaching and printing and dyeing processes. The following are some applications of ammonium pe rsulfate in the textile industry:
rsulfate in the textile industry:
Bleach: Ammonium persulfate is often used as an oxidant for textile bleaching. In textile production, common raw materials such as cotton, linen, wool, etc. may contain impurities, pigments, or other unclean substances during growth or processing. The oxidizing properties of ammonium persulfate enable it to effectively oxidize and remove these impurities, thereby improving the cleanliness and whiteness of textiles.
Printing and dyeing process: Ammonium persulfate is also widely used in the printing and dyeing process of textiles. In dyeing and printing, ammonium persulfate acts as an oxidant to participate in the reaction, helping the dye bind to the fibers, ensuring uniform and long-lasting color. Its properties as an oxidant make it an effective auxiliary in the field of printing and dyeing.
Reaction condition control: When using ammonium persulfate in the textile industry, it is necessary to carefully control the reaction conditions, including temperature, pH value, etc. This helps to ensure the effectiveness of oxidation reactions and the quality of textiles. At the same time, the amount of ammonium persulfate used should also be reasonably controlled to avoid possible side reactions and excessive oxidation.
Environmentally friendly: Compared to some traditional bleaching and oxidizing agents, ammonium persulfate has better environmental friendliness in the textile industry. The main decomposition products are sulfuric acid and ammonia, which are relatively easy to handle.
3. Electronic manufacturing
In the field of electronic manufacturing, ammonium persulfate is commonly used as a special etchant for etching circuit boards (PCBs). The following is about the application of ammonium persulfate in electronic manufacturing:
Circuit board etching: Ammonium persulfate is widely used in the etching process when manufacturing circuit boards. In electronic devices, circuit boards are the foundation for connecting and supporting electronic components, and etching is one of the key steps in making circuit boards. Ammonium persulfate, through its strong oxidizing properties, can effectively remove unwanted copper layers during the etching process, forming the desired circuit patterns.
properties, can effectively remove unwanted copper layers during the etching process, forming the desired circuit patterns.
Etching process: In circuit board etching, the oxidizing property of ammonium persulfate enables it to oxidize copper, generating soluble copper ions. These copper ions will then be dissolved to achieve the purpose of etching. This process is usually carried out under certain temperature and acidity conditions and requires careful control to ensure the accuracy of etching.
Effect control: As an etchant, ammonium persulfate requires strict control over the etching effect in electronic manufacturing. This includes controlling the etching rate, ensuring etching uniformity, and preventing the generation of poor etching by-products. Accurate etching is the key to ensuring the quality and performance of circuit boards.
Environmentally friendly: Ammonium persulfate is relatively environmentally friendly, and its decomposition products are mainly sulfuric acid and ammonia. In electronic manufacturing, environmentally friendly chemical selection is crucial for meeting environmental standards.
Overall, the application of ammonium persulfate in electronic manufacturing mainly focuses on the etching process of circuit boards. Through its strong oxidizing properties, it can provide an effective way to manufacture complex circuit board structures, thereby supporting the manufacturing of modern electronic devices. When using ammonium persulfate, precise control is required based on specific process requirements and etching parameters to ensure high quality and efficiency in circuit board manufacturing.
4. Application in hair whitening
Ammonium persulfate is widely used in hair whitening as a powerful oxidant that can help remove pigments from hair and achieve bleaching effects. The following are the main applications of ammonium persulfate in hair whitening:
Bleaching effect: Ammonium persulfate, as an oxidant, can oxidize pigment molecules in hair, causing them to lose color and achieve a bleaching effect. This is very effective for changing hair color, repairing dyeing errors, or achieving special hair color effects.
Efficiency: Ammonium persulfate hair bleach has a high bleaching efficiency and can achieve significant bleaching effects in a relatively short period of time. This makes it one of the popular hair bleaches in the beauty industry.
Adjusting hair color: Ammonium persulfate bleach is commonly used in the hair industry to mix with dye after bleaching to adjust hair color. By using appropriate mixing ratios, various hair colors can be customized to meet the individual needs of customers.
Easy to use: Ammonium persulfate hair bleach is usually sold in the form of powder or lotion, which is relatively convenient to use. Hairdressists can choose suitable bleaching agents based on customer needs and hair quality, and achieve ideal bleaching effects through professional operating techniques.
Attention: When using ammonium persulfate hair bleach, it is necessary to control the concentration, action time, and temperature during use of the bleach to ensure uniform bleaching effect and minimal hair damage. In addition, it is important to have a thorough understanding of the customer's hair condition to avoid excessive use of hair bleaching agents that can cause significant damage to the hair.
THE ROLE OF PCB ETCHING:
Ammonium persulfate plays a crucial role in the etching process of printed circuit boards (PCBs). As an etchant, ammonium persulfate is used in electronic manufacturing to remove unwanted copper layers and form the required conductive patterns on circuit boards. The following are the main functions and characteristics of ammonium persulfate in PCB etching:
Copper oxide layer: Ammonium persulfate serves as a strong oxidant and can undergo oxidation reaction with copper. In PCB etching, its oxidizing properties enable it to effectively oxidize the copper layer and convert it into soluble copper ions.
Etching rate control: By adjusting the concentration, temperature, and other operating para meters of ammonium persulfate in the etching solution, the etching rate can be controlled. This flexibility enables manufacturers to achieve precise etching, ensuring the formation of precise circuit patterns.
meters of ammonium persulfate in the etching solution, the etching rate can be controlled. This flexibility enables manufacturers to achieve precise etching, ensuring the formation of precise circuit patterns.
Etching uniformity: Ammonium persulfate has good uniformity during the etching process, which can ensure consistent etching effect on the entire surface of the circuit board. This is crucial for the quality and performance of the circuit board.
Environmental friendliness: Ammonium persulfate is relatively more environmentally friendly, and its decomposition products are mainly sulfuric acid and ammonia, which are easier to handle compared to some other etchants.
Safe operation: When using ammonium persulfate for PCB etching, corresponding safety measures need to be taken, including wearing gloves, goggles, etc., to prevent splashing or direct contact with the human body.
WATER TREATMENT APPLICATIONS:
Ammonium persulfate has certain applications in the field of water treatment, mainly involving its oxidation properties and characteristics of sterilization and disinfection. The following are some main applications of ammonium persulfate in water treatment:
Oxidative water treatment agent: Ammonium persulfate is a strong oxidizing agent that can be used in water treatment to oxidize and remove organic matter, sulfides, and some difficult to degrade wastewater components. It can promote the degradation of organic matter in wastewater and help improve water quality.
Bleach: Ammonium persulfate is often used as a bleaching agent in water treatment due to its strong oxidizing properties. In some water treatment processes, such as sewage treatment and drinking water purification, it can help remove colors and odors from the water.
Sterilization and disinfection: The oxidizing nature of ammonium persulfate gives it a certain degree of sterilization and disinfection effect. It can be used in water treatment processes to help control microorganisms in water, reduce the number of bacteria, algae, and other microorganisms, and ensure that water quality meets specified hygiene standards.
Deodorant: Ammonium persulfate can also be used as a deodorant in water treatment. Its oxidizing properties can eliminate certain organic pollutants in water, reduce the problem of water odor, and improve the taste of water.
Solid wastewater treatment: In some solid wastewater treatment processes, ammonium persulfate can serve as an auxiliary treatment agent to help degrade and remove organic waste in water, improving the treatment efficiency of wastewater.
MEDICAL AND LABORATORY USE:
Ammonium persulfate has some specific uses in the medical and laboratory fields, mainly involving its oxidation properties and applications in chemical experiments. The following are some common uses of ammonium persulfate in medical and laboratory settings:
Oxidizing agent in the laboratory: Ammonium persulfate is a strong oxidizing agent commonly used in chemical laboratories. It participates in redox reactions and is used as an initiator to promote some organic synthesis reactions. Its oxidative properties make it an important reagent in the laboratory.
Biochemical research: Ammonium persulfate may be used in specific experiments such as protein analysis and modification in biochemical research. However, caution should be taken when using it as its strong oxidizing properties may have an impact on biomolecules.
Protein electrophoresis: In protein electrophoresis experiments, ammonium persulfate can be used as a source of ammonium sulfate ions, which helps maintain the pH value of the electrophoresis buffer to ensure the stability of electrophoresis.
DNA gel electrophoresis: Ammonium persulfate can also be used in DNA gel electrophoresis, as one of the components of electrophoresis buffer, to help maintain the pH value and conductivity of gel.
Drug preparation: In some special pharmaceutical preparation processes, ammonium persulfate may act as an oxidant or initiator and participate in certain organic synthesis reactions. The application in this area requires careful operation in the pharmaceutical field to ensure the purity and quality of drugs.
ENVIRONMENTAL EFFECT:
Operation and Storage Guidelines:
Ammonium persulfate, as a chemical substance, its use and treatment may have a certain impact on the environment. The following are the possible environmental impacts of ammonium persulfate:
Water pollution: In some application scenarios, ammonium persulfate may be discharged into the water through wastewater, and its oxidizing properties may have an impact on the ecosystem in the water. Excessive ammonium persulfate may lead to oxidation reactions in water and have adverse effects on aquatic organisms.Air pollution: In some industrial production processes, the dust or vapor of ammonium persulfate may be released into the air, which has a certain impact on the surrounding air quality. Ammonium persulfate in the air may participate in redox reactions, producing harmful gases.
Soil impact: If ammonium persulfate is released in large quantities into the soil, it may cause certain changes in the chemical properties of the soil. This may affect the microbial community and plant growth in the soil.
Toxicity: Ammonium persulfate itself has certain toxicity, especially at high concentrations. If used or disposed of under improper conditions, it may have direct toxic effects on organisms in the ecosystem.
To reduce the potential impact of ammonium persulfate on the environment, the following measures need to be taken:
Reasonable use and disposal: Ensure appropriate protective measures are taken when using ammonium persulfate to avoid excessive use. Waste should be properly disposed of in accordance with local environmental regulations.
Prevent leakage: Take measures to prevent the leakage of ammonium persulfate during production, transportation, and storage to prevent it from entering the environment.
Environmental monitoring: Regularly monitor the concentration of ammonium persulfate in the environment to take timely measures to address potential environmental issues.
Alternative research: Where possible, seek more environmentally friendly and safer alternatives to reduce negative impacts on the environment.
SAFETYMEASURESANDTREATMENTOFAMMONIUMPERSULFATE:
Ammonium persulfate is an important chemical substance, but a series of safety measures must be followed during use and treatment to ensure personnel safety and environmental protection. The following are detailed guidelines on safety measures and proper handling of ammonium persulfate:
1.Storage conditions
Store ammonium persulfate in a dry, cool place, away from sources of fire and combustibles.
Avoid mixing with reducing agents, organic substances, etc. to reduce potential hazardous reactions.
2. Personal protection
Use appropriate protective equipment, including goggles, chemical protective clothing, and gloves.
Avoid direct skin contact to prevent allergic reactions or irritation.
3. Processing and operation
Wear appropriate respiratory protective equipment during operation, especially in situations where gas, vapor, or dust may be generated.
Avoid inhaling ammonium persulfate dust and use a local ventilation system to ensure good indoor ventilation.
4. Emergency response
If spillage or leakage occurs, take emergency measures immediately, including wearing appropriate personal protective equipment and isolating the leakage area.
Use absorbent agents (such as sand or desiccants) to quickly absorb the leaked material and prevent diffusion.
5. Waste disposal
Abandoned ammonium persulfate should comply with local regulations and environmental standards.
Do not dump waste into water bodies or discharge it into sewers to avoid environmental pollution.
6. First aid measures
After contact with ammonium persulfate, immediately rinse the skin or eyes with plenty of water. If necessary, seek professional medical assistance.
If ingested, drink plenty of water immediately and seek medical attention immediately.
7. Preventing fires
Ammonium persulfate itself is not flammable, but it may decompose and release toxic gases at high temperatures. Therefore, avoid high temperatures and sources of ignition.
Carbon dioxide, foam or dry powder fire extinguishers can be used during fire extinguishing.
8. Regular training
Regular training should be provided to personnel handling ammonium sulfate, including safety operating procedures, emergency response, and personal protective requirements.
SAFEAPPLICATIONOFAMMONIUMPERSULFATEINTHELABORATORY:
Ammonium persulfate is a commonly used oxidant used in many industrial and laboratory applications. However, it is a chemical with strong oxidizing properties, so some safety measures need to be taken during processing and storage. The following are safety measures and handling suggestions for ammonium persulfate:
Personal protective measures:
Wear acid and alkali resistant gloves, goggles, and a protective face mask to prevent ammonium persulfate from coming into contact with the skin, eyes, or respiratory tract.
Wear appropriate protective clothing to minimize exposed skin.
Ventilation:
Maintaining good ventilation during the treatment of ammonium persulfate can reduce gas concentration by using ventilation systems or operating outdoors.
Avoid mixing:
Avoid mixing ammonium persulfate with flammable substances or other chemicals to prevent dangerous reactions.
Prevent inhalation:
Avoid inhaling dust or gas containing ammonium persulfate. Protective masks or respirators should be used during operation.
Storage requirements:
Store ammonium persulfate in a dry, cool, and well ventilated place, away from sources of fire and combustibles.
Avoid storing together with organic matter, reducing agents, or flammable substances.
First aid measures:
If skin contact occurs, immediately rinse the affected area with plenty of water.
When in contact with the eyes, rinse them quickly with plenty of water and seek medical assistance.
When inhaling ammonium persulfate, it should be immediately transferred to a fresh air area and artificial respiration should be performed if necessary.
Waste disposal:
Abandoned ammonium persulfate should be disposed of in accordance with local regulations and rules. Usually, it should be handed over to qualified waste disposal units for disposal.
EXAMPLES OF CONSUMER PRODUCTS CONTAINING AMMONIUM PERSULFATE:
Ammonium persulfate is often used in some consumer products, mainly as a bleaching agent, oxidant, or additive. Here are some examples of consumer products that may contain ammonium persulfate:
Bleach:
Some clothing bleaches and detergents may contain ammonium persulfate, which is used to remove stains and bleach from clothing.
Dough improver:
In food processing, ammonium persulfate can be used as a dough improver in pastry products such as bread and cakes.
Toothpaste:
Some toothpaste may contain ammonium persulfate for special cleaning or bleaching purposes.
Shampoo and hair care products:
In some shampoo and hair care products, ammonium persulfate may be used as a bleach or other treatment agent.
Water treatment agent:
In some household water treatment systems, ammonium persulfate can be used as an oxidant to remove impurities from water.
Rubber products:
In the production process of rubber products, ammonium persulfate can be used as a vulcanizing agent, which helps with the crosslinking and curing of rubber.
INDUSTRY APPLICATIONS:
Ammonium persulfate is mainly used in various industrial and laboratory applications, including rubber vulcanization, pulp bleaching, water treatment, and dough improvers in pastry production.
Regional distribution: The production and use of ammonium persulfate are distributed globally. The main production countries include China, the United States, and some European countries. China is one of the main producers of ammonium persulfate.
Market driving factors: Industry development, growth of industrial production, and gradual improvement of environmental regulations may affect the demand for ammonium persulfate in the market.
Industry trend: In the rubber industry, the application of ammonium persulfate may be influenced by factors such as increased demand for rubber products and the development of the automotive industry.
In terms of environmental protection, the demand for renewable energy and environmentally friendly water treatment may also affect the market for ammonium persulfate.
Price fluctuation: The price of ammonium persulfate may fluctuate due to various factors such as raw material costs, supply and demand relationships, and changes in the international market.
Renewable energy industry: Ammonium persulfate can be used in certain renewable energy fields, such as the preparation of fuel cells, which may drive demand in the renewable energy industry.
Market competition: There are multiple manufacturers and suppliers in the ammonium persulfate market. Market competition may involve factors such as product quality, innovation capability, and pricing strategy.
CONCLUSION:
Through this article, we have gained an in-depth understanding of the diverse uses of ammonium persulfate, from industry to hair care products, to medicine and laboratories. We discussed its manufacturing process, safety, environmental impact, and comparison with other chemical substances. The case study provides readers with practical application demonstrations, while also pointing out the challenges and limitations faced by ammonium persulfate.
When using ammonium persulfate, safety is the primary consideration. Following the correct operating procedures and safety measures is crucial for maximizing its effectiveness. At the same time, we should also recognize the environmental impact of ammonium persulfate and encourage the search for more environmentally friendly alternatives.
English
العربية
Français
Русский
Español
Português
Deutsch
italiano
日本語
한국어
Nederlands
Tiếng Việt
ไทย
Polski
Türkçe
አማርኛ
ភាសាខ្មែរ
Bahasa Melayu
ဗမာစာ
Filipino
Bahasa Indonesia
magyar
Română
Čeština
қазақ
हिन्दी
فارسی
Kiswahili
Slovenčina
Slovenščina
Norsk
Svenska
українська
Ελληνικά
Suomi
עברית
Latine
Dansk
اردو
বাংলা
Hrvatski
Afrikaans
Eesti keel
සිංහල
latviešu
Български
Hausa
íslenska
Kurdî
Lietuvių
isiZulu














